Life
- what is it made of?
The Importance of Carbon
|
Life, as we know it, depends on the existence of large molecules built around chains of carbon atoms. However, living organisms are mostly water (oxygen and hydrogen). Carbon and these two elements, along with nitrogen, phosphorus, calcium and sulfur, can form the bulk of the compounds found in living things. Other trace element are needed in very small quantities as well. Altogether, human beings depend on about 27 different chemical elements, while some bacteria make do with as few as 17, and viruses with even fewer. The overall chemical makeup of most living things is similar to that of the Earth's oceans. But living things differ in chemical content from the Earth's crust most obviously, in making very little use of silicon, which is a major component of rocks. The Universe as a whole, though, is mostly hydrogen with about 10% helium and small amounts of all the other elements. Recent findings by radio astronomers have shown concentrations of water and organic molecules, in vast clouds within our galaxy. These are the essential building blocks of life and this adds credence to the idea that life might exist, and even, originate in deep space. The most important criteria for life, as well as being the most fundamental, is the ability to organize into repetitive structures at different orders of complexity - molecular and cellular for instance. With carbon, molecular chains, known as Linear Polymeric Molecules (LPM), can form and these are the key to replication and the conservation of the creation instructions. We know these molecules better as DNA, RNA and peptides. Other elements can fulfill the role of being the base in an LPM, too, but carbon has some unique properties. Why is this?
An LPM base element must have at least three bonds - one to link to the base atom to its right and one to its left - forming the backbone; and another to the side group - made up of co-atoms or molecules; this forms the organic compound. It is possible to have more than three bonds, but for reasons of physical space, no more than four bonds on each base atom are realistically possible. Carbon is unique in that it forms only four bonds. Other candidates for the chain backbone - silicon, boron or sulfur, for instance - can form inconsistent numbers of bonds - something between three and six, so replication and growth processes are unreliable.
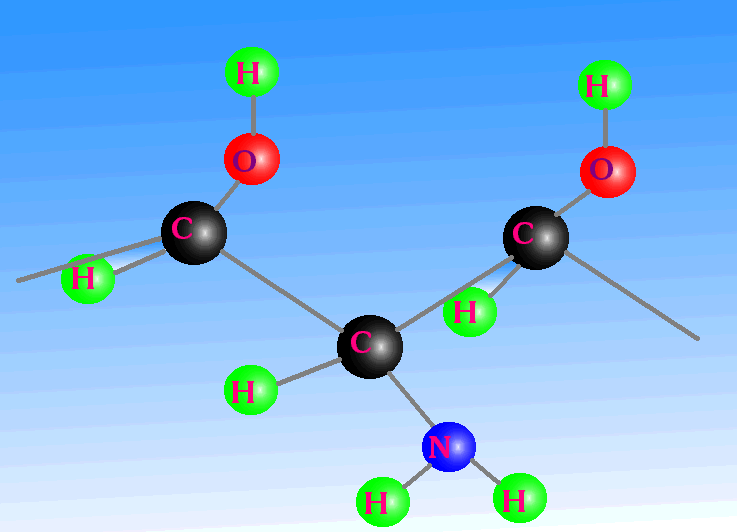
Additionally, the primary carbon-carbon bond is twice as strong as a silicon-silicon bond and also strong compared with its secondary link to the side group. This keeps the backbone of the structure in place, even under fluctuating temperatures; whilst at the same time allowing chemical reactions to occur on these secondary links. Other base-element candidates do not possess such properties. In this picture we can see a carbon chain backbone in red. Attached to this are groups of atoms and molecules - OH, NH2, OH. Note how they point out into the environment, protecting the carbon chain on the one hand, yet being able to interact with the environment on the other. Carbon can make double bonds as well as single bonds, which is another very important and unique characteristic of carbon. If several double-bonded atoms are close together, then they can share electrons in a process called resonance. Such molecules have a very special ability: They can absorb light from the infrared through to, and including, the ultraviolet. Photosynthesis and sight are two consequences of this, and the two chemicals involved are chlorophyll and retinol, respectively. |
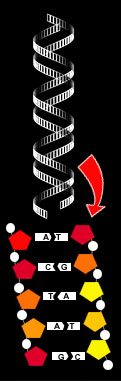
DNA Molecule The carbon chain is able to link to a wide range of chemical elements and molecules. This, combined with carbon's chaining ability, enables the synthesis of organic molecules that constitute metabolism in its simplest form. This provides an enormous diversity of molecules based on this simple structure. Thousands of molecules are possible using just carbon and hydrogen. When oxygen, nitrogen, calcium, phosphorus and sulfur are included, this rises to tens of millions. Importantly, from the life perspective, some such molecules have shapes and chemistries that allow them to replicate and to serve as catalysts in other chemical reactions. One such, is DNA. As we have seen, carbon and its associated "elements of life" can make up a vast range of molecules, many of them highly complex. This branch of chemistry is therefore an important one and it has its own name - organic chemistry, so called because it deals mainly with the the chemistry of life. It plays an important role in economic activity, particularly in the manufacture of industrial chemicals and pharmaceuticals, all branches of medicine and in food production, both on the farm and in the factory. Additionally, because of the geometry of the way carbon works as the backbone of organic molecules, it is possible to create molecules that are right- or left-handed. This ability is called chirality. It should be noted that not all organic molecules are chiral: some can be symmetrical. The chiral molecule, however, has properties that are crucial to the working of the chemistry of life. As far as life on Earth is concerned, all normal chiral molecules associated with life processes are left-handed. In terms of basic chemistry, left- and right- chiral molecules are identical, but their behavior can be vastly different. This is because the chemistry of life depends on the ability of molecules to form atomic bonds at several points simultaneously, to create new compounds. The molecules, however, are three-dimensional structures and to make the bonds work they must be turned round and fitted, much as a jigsaw puzzle would be put together. The consequence is that molecules must be the correct shape, in order to form the required bonding, as well as being chemically correct. Players of the computer jigsaw puzzle game, Tetris, will appreciate the problem. Some pieces are handed - they are chiral - and however they are turned around and manipulated, they will not fit into the available slots. In fact Tetris would soon become incredibly and boringly easy, if the chiral pieces were not present. So, in terms of bio-chemistry, you can see that it is impossible for the "wrong-handed" molecule to be used in a particular process, even if it is chemically correct. This characteristic of some organic molecules is key in the processes of life, particularly in replication, such as in the behavior of DNA. Let us now return to the humble carbon atom and think about its role when considering life elsewhere, off planet Earth. The unique characteristics of carbon give it a versatility and functionality like no other atom in the periodic table. These are why carbon is the basis of life here. Additionally, carbon is only beaten in abundance by hydrogen, helium and oxygen, at least within the Solar System. It is ten times more abundant than silicon and a million times more abundant than boron, which have also been suggested as alternative base elements for living molecules. In interstellar space, radio astronomers have detected just over eighty different molecular compounds. Over seventy of these contain carbon, while only eight contain silicon. The chemical characteristics of carbon are of course, universal, so apply as much in distant galaxies as they do here. As the abundance of carbon appears to be similar out in deep space too, then carbon must be the best candidate as the basis of life everywhere. |
|
|
But carbon is not necessarily the key factor...
|
The
cell
Life:
What Exactly Is It?- Discussion with Dr Stanley Miller
Life
in the Universe
Nasa
- Origins Investigation 13 Characterize the traits of the universal common
NASA
Research Findings Space Science Workshop 1996
Evolution
and the Origins of life
From
Primordial Soup to the Prebiotic Beach
Go
to
Home
| Space Station
| Mars | Rainforest
© 1999 Satellite Events Enterprises Inc.