




Titan is the only large moon in the Saturnian system and it is the only moon in the Solar System with a thick atmosphere. Not only that, the atmosphere shows signs of containing many elements and compounds that are the basis of organic chemicals. For this reason it is a very interesting place in our the Search for Life. As Saturn is twice as far from the Sun as is Jupiter, it receives only one-quarter of the solar radiation that Jupiter receives. This means it is very very cold here, but because the atmosphere of Titan is so thick it absorbs whatever solar radiation is available and uses it to drive chemical processes in the atmosphere and on the surface. This means that weather and complex chemistry occur on Titan, even if this is rather slow.

The depth of its atmosphere is ten times Earth's, due not only to the higher pressures, but also the low temperatures and low gravity. The atmosphere is extremely dense and an opaque layer of haze, about 200 km (125 miles) above the surface, effectively obscures any attempt at optical observation. In fact it is the only major body in the Solar System whose surface we have never observed at high resolution. Within the last few years, though, the Hubble Space Telescope infra-red cameras have been able to penetrate the cloud and we now have some coarse resolution images, that show the largest features on the surface.

Images of Titan were taken by the Voyagers. All they showed was a uniformly-smooth orangey ball - the dense atmosphere. Spectral analysis, both from the spacecraft and from Earth, revealed the atmosphere to be largely Nitrogen - 97%. Trace amounts of a cocktail of organic gases are present, and these give Titan its characteristic color - Methane, Ethane, Ethene, Propane and half-a-dozen others. Hydrogen and Carbon Mon- and Di-oxide are also there and significant amounts of water vapor were detected by ESA's Infrared Space Observatory in 1997. The organic compounds are in a reduced rather than oxidized form. Also, and this came as a surprise to many planetary scientists, the nitrogen is not contained in the form of ammonia as it is in the gas giant planets - Jupiter and Saturn.
At present, two explanations are offered for the lack of ammonia. The first suggests that when Titan formed it was so cold that all the nitrogen was locked up in various ices as a clathrate - a solid material with tiny cavities containing the gas at the atomic level. On melting through radiogenic (heat produced through the radio-active decay of heavy elements such as uranium), or by tidal heating, the nitrogen was released. Ammonia must also have been present, but this has not been released in this way.
The second explanation, which is most favored at present, suggests an unstable active atmosphere that recycles the nitrogen though the agency of ultraviolet solar radiation. This liberates hydrogen which then escapes into space. There is good evidence for this, as Titan orbits in a thin halo of hydrogen. This was detected by the Voyagers. The process is time-limited, because Titan has only so much hydrogen, so once this is all liberated to space the chemistry will cease.
The mechanism works through ultraviolet disassociation of the ammonia. Basically, one of the three hydrogen atoms gets knocked off the ammonia - NH3 - by a photon. This leaves a radical, NH2 and the single H escapes away to space.

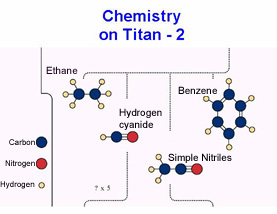
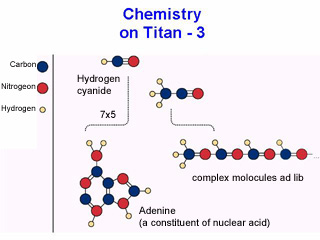
Through a complex chain of chemical processes, various organic hydrocarbons found in the atmosphere are produced through a myriad of chemical reactions between the NH radicals and carbon mon- and di-oxide. The processes also provide for the possibility of long carbon chain molecules forming and these could produce interesting organic compounds, which in some cases could be the precursors of life.
All this complex chemistry suggests that the chemical precursors of life are present on Titan. However, Titan's problem for life may be that it is, and always was, too cold for life as we understand it to exist. Titan is regarded, by optimistic life-scientists and planetary specialists, as a natural laboratory to study the origins of life. It may be in a state like the Earth was four-billion or more years ago when similar organic chemistry was taking place here, though it will be much colder.
Conditions on Titan are such that methane could exist in all the three physical states: vapor, liquid and solid, for the temperatures and pressures are close to methane's triple-point. It has been speculated that if ammonia rain and snow fall, great ice cliffs could form near the poles and oceans may exist.

The Hubble Space Telescope has been able to map Titan, and has found highlands and lowlands, but does an ocean of liquid ammonia exist?
If life did exist on Titan, what might it be like? Titan's chemistry may be active enough to be able to create amino acids, and other basic building blocks of organic chemistry may be present. The big problem for life at these low temperatures and low energy levels is the rate of the process. The speed of chemical reactions drop off exponentially with temperature. If life exists on Titan, then the rate of metabolism will be inordinately slow, which raises the key question "Will it therefore be detectable?"
That is very difficult to answer, not only because of the slow rates of reaction and process, but because it is highly unlikely that liquid water could be present. This is unlike the case of Europa, where temperatures could be higher. But it is interesting to note that ammonia - NH3 - is a polar solvent like water, but only liquid at much lower temperatures than occur on Earth. Titan is the one place we know of where this may occur and ammonia could take the place of water as the life-giving solvent. There is a problem, though.
Water has another highly unique characteristic. It assists in the working of enzymes, which are organic catalysts. Enzymes are critical in allowing amino acids, the main building-blocks of life, to react with other chemicals both quickly and efficiently.
Additionally, nearly all liquids as they solidify do so from below. Generally, the solid state of a substance is denser than the liquid state. So assuming the presence of a gravitational field, when a solid forms or solidifies it does so on the bottom. Water is virtually unique in the way it solidifies. It forms ice and this floats on the surface of the liquid. A layer of ice creates an insulating blanket, which assists in preventing water below from freezing as well, unless very much colder conditions are encountered (ie, much colder than would be necessary to freeze bottom-solidifying material).
This allows living organisms to continue functioning below a water-ice layer, while conditions above are too severe for survival. In the Arctic for instance, the air temperature may be sixty below, but the water temperature under the ice is a pleasant freezing point, more or less. Similarly, ice will melt fairly readily when warmer conditions prevail. Bottom-forming solids may never melt out (convection heat tends to rise), but continue to build up by solid layering, as warm and cold conditions cycle. Ammonia is a bottom-solidifying material and this factor would not help life to flourish on Titan.
Go
to
Home
| Space Station
| Mars | Rainforest
© 1999 Satellite Events Enterprises Inc.